Estrogen Interactions with Warfarin: What You Need to Know About Blood Thinners and Hormones
Feb 7 2026
When your doctor prescribes a biologic drug like Humira or Enbrel, you might be offered a biosimilar substitution, a highly similar version of a brand-name biologic drug approved after the original patent expires. Also known as biogeneric, it works the same way in your body but costs significantly less. Unlike regular generics, which are simple chemical copies, biosimilars are made from living cells and are incredibly complex to replicate. That’s why they’re not exact duplicates—but they’re proven to be just as safe and effective, according to the FDA and major medical groups.
Biosimilars, medications designed to match the clinical performance of existing biologics are already replacing expensive drugs for conditions like rheumatoid arthritis, Crohn’s disease, and certain cancers. Many insurance plans now push for biosimilar substitution, the process of swapping a brand-name biologic for its biosimilar version at the pharmacy to cut costs. This isn’t just about saving money—it’s about making life-saving treatments accessible to more people. In fact, one study found that biosimilar use in the U.S. saved over $13 billion in just five years.
But here’s the catch: not all states allow pharmacists to switch your drug without telling your doctor. Some require prior authorization, or even your explicit consent. And while most people tolerate biosimilars just fine, a few report minor differences in how they feel—usually because of the delivery method or inactive ingredients, not the active drug. That’s why knowing your rights and asking questions matters. You don’t have to accept a switch blindly. Your doctor, pharmacist, and insurance company all play a role in this process.
What you’ll find below are real stories and practical guides from people who’ve been through this. You’ll learn how to spot when a switch is being proposed, what questions to ask your pharmacist, how to challenge an unwanted substitution, and why some patients end up better off after switching. You’ll also see how formularies, insurance rules, and patent laws shape what drugs end up on your shelf. Whether you’re paying out of pocket or through Medicare, this isn’t just technical jargon—it’s your money and your health on the line.
Interchangeable biosimilars can be swapped automatically at U.S. pharmacies, but only if FDA-approved and allowed by state law. Learn how this affects cost, safety, and patient access to biologic drugs.
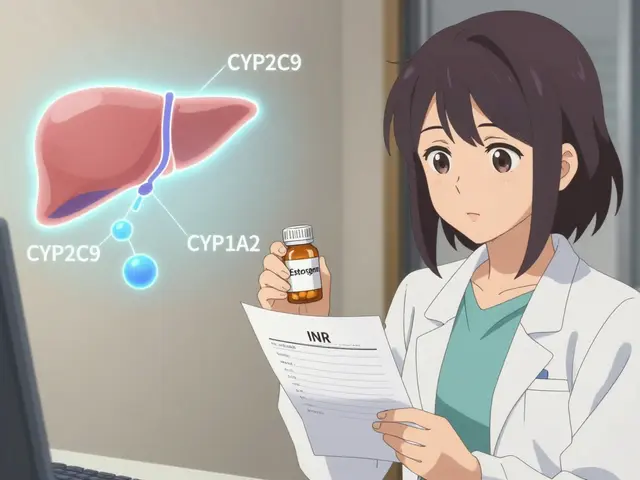
Feb 7 2026
Mar 4 2025
Oct 20 2025

Dec 9 2025

Mar 15 2025