COVID-19 Antiviral Comparison Tool
Recommended Treatment Option
Why This Choice?
| Drug | Administration | Efficacy (Reduction in Hospitalization) | Duration | Special Considerations |
|---|---|---|---|---|
| Molnupiravir | Oral pill | ~30% | 5 days | Not for pregnancy; requires eGFR >30 mL/min |
| Paxlovid | Oral pill with ritonavir booster | ~89% | 5 days | May interact with many medications; avoid with certain statins |
| Remdesivir | IV infusion | ~30% (early hospital cases) | 3 days | For early hospital admission; needs clinical supervision |
| Monoclonal Antibodies | IV infusion or subcutaneous injection | >90% (pre-variant) | Single dose | Variant-sensitive; requires infusion center |
Molnupiravir (Lagevrio)
Works by: Introducing mutations into viral RNA, causing "error catastrophe".
Usage: For non-hospitalized adults with mild-to-moderate symptoms at risk of severe disease.
Side Effects: Mild nausea, dizziness.
Contraindications: Pregnancy, severe renal impairment.
Paxlovid
Works by: Inhibiting viral protease enzyme.
Usage: For high-risk patients within 5 days of symptom onset.
Side Effects: May interact with many medications.
Contraindications: Severe liver disease due to ritonavir component.
Remdesivir
Works by: Inhibits viral RNA synthesis.
Usage: For early hospital admissions via IV infusion.
Side Effects: Generally well-tolerated.
Contraindications: Not suitable for outpatient use.
Monoclonal Antibodies
Works by: Neutralizing spike protein to block viral entry.
Usage: For early outpatient treatment with high efficacy.
Side Effects: Rare allergic reactions.
Contraindications: May lose effectiveness against new variants.
When the omicron wave surged in 2022, a new pill called Molnupiravir is an oral antiviral that works by forcing the virus to make copying errors in its RNA. Fast, easy to swallow, and shelf‑stable, it looked like the perfect home‑treatment for COVID‑19. But the market soon filled with other options - from the blockbuster combo Paxlovid to IV‑only drugs like Remdesivir. If you’re wondering which drug actually delivers the most benefit, this side‑by‑side look breaks down the science, the real‑world data, and the practical details that matter to patients and clinicians alike.
TL;DR
- Molnupiravir reduces hospitalisation by ~30% in high‑risk adults, while Paxlovid hits ~89%.
- Paxlovid requires a 5‑day course with a boosted‑ritonavir component; Molnupiravir is a simple 5‑day pill.
- Remdesivir is IV, given over 3days, and best for early hospital admission.
- Monoclonal antibodies offer >90% protection but need infusion and can lose efficacy against new variants.
- Choice hinges on timing, drug interactions, kidney/liver health, and local availability.
Understanding Molnupiravir
Molnupiravir, marketed as Lagevrio, received emergency use authorization (EUA) from the FDA in December2021. It works by inserting a nucleoside analogue (NHC) into the viral RNA polymerase, causing "error catastrophe" - essentially overwhelming the virus with mutations.
Key attributes:
- Form: 200mg capsule, taken twice daily for five days.
- Indication: Non‑hospitalised adults with mild‑to‑moderate COVID‑19, at risk of severe disease.
- Efficacy: In the phase‑3 MOVe‑OUT trial, 30% relative reduction in hospitalisation or death versus placebo.
- Side‑effects: Mild nausea, dizziness, and rarely, urinary discomfort.
- Contra‑indications: Pregnancy (mutagenic risk) and severe renal impairment (eGFR<30mL/min).
Because it’s metabolised chiefly by hepatic enzymes, drug‑drug interactions are modest - a plus for patients on many prescriptions.
Major Alternatives on the Market
Four other therapies dominate the COVID‑19 antiviral landscape in 2025. Each has a distinct mechanism, route, and evidence base.
Paxlovid (nirmatrelvir/ritonavir)
Paxlovid combines nirmatrelvir, a protease inhibitor that blocks the SARS‑CoV‑2 main protease (Mpro), with ritonavir, a CYP3A4 booster that prolongs nirmatrelvir’s half‑life.
- Form: Two 150mg nirmatrelvir tablets plus one 100mg ritonavir tablet, taken twice daily for five days.
- Efficacy: 89% relative reduction in hospitalisation/death (EPIC‑HR trial).
- Side‑effects: Bitter taste, diarrhoea, and occasional dysgeusia.
- Drug interactions: Strong CYP3A4 inhibitors/inducers require dose adjustment or avoidance.
Remdesivir (Veklury)
Originally the first FDA‑approved COVID‑19 drug, Remdesivir is a nucleoside analogue that stalls the viral RNA‑dependent RNA polymerase.
- Form: Intravenous infusion, 200mg on day1 then 100mg daily for 2-5days.
- Efficacy: In the ACTT‑1 trial, 87% faster recovery in hospitalized patients, but modest impact on mortality.
- Side‑effects: Elevated liver enzymes, infusion‑site reactions.
- Use case: Early hospital admission or high‑risk outpatients unable to take oral meds.
Monoclonal Antibody Therapies
Products like Sotrovimab and newer broadly neutralising antibodies bind the spike protein, preventing viral entry.
- Form: Single IV or sub‑cutaneous injection (usually 500mg).
- Efficacy: Up to 93% reduction in progression when given within five days of symptom onset.
- Limitations: Loss of activity against certain Omicron sub‑variants, high production cost, and infusion logistics.
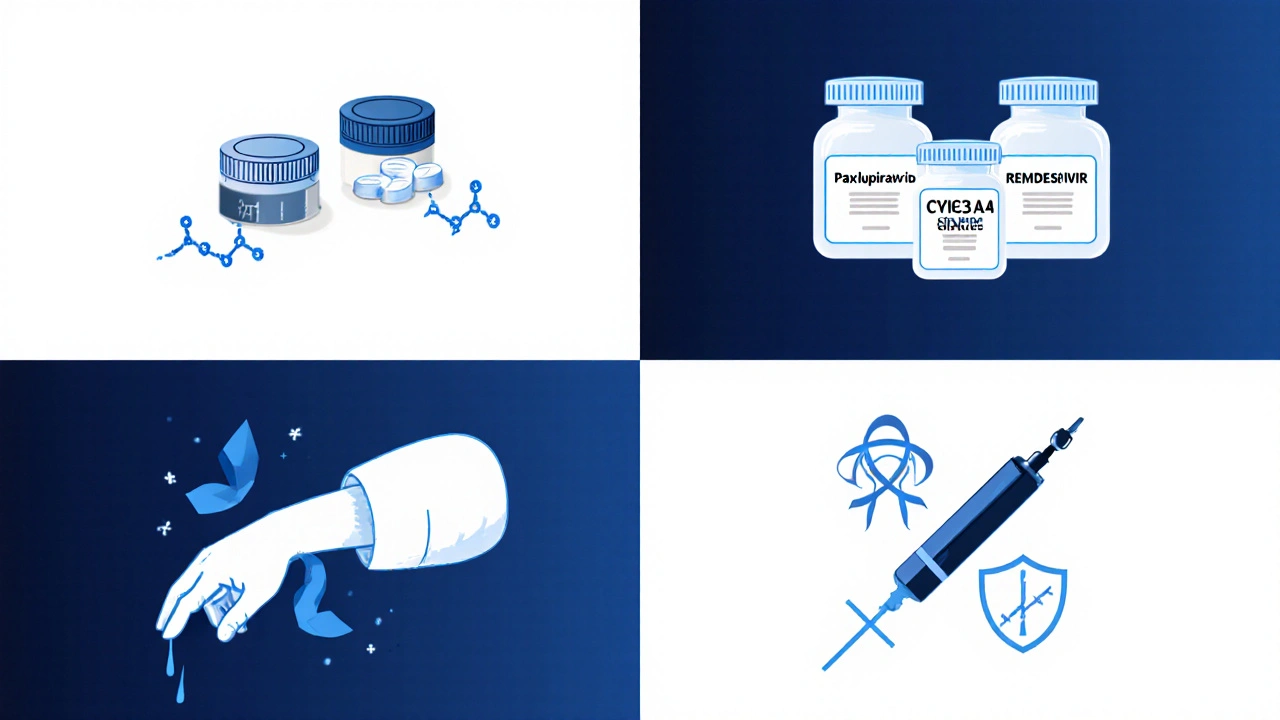
Side‑by‑Side Comparison
| Drug | Mechanism | Route & Duration | Hospitalisation Reduction | Common Side‑effects | Approval Status (US/EU) |
|---|---|---|---|---|---|
| Molnupiravir | Error‑inducing nucleoside analogue | Oral, 5‑day course (200mg BID) | ~30% (MOVe‑OUT) | Nausea, dizziness | EUA (US), Conditional (EU) |
| Paxlovid | Protease inhibition (Mpro) | Oral, 5‑day course (nirmatrelvir+ritonavir) | ~89% (EPIC‑HR) | Altered taste, diarrhoea | Full approval (US, EU) |
| Remdesivir | RNA‑polymerase chain terminator | IV, 3‑5day infusion | ~30% faster recovery (ACTT‑1) | Liver enzyme rise, infusion‑site pain | Full approval (US, EU) |
| Monoclonal Antibodies | Spike‑protein neutralisation | IV or SC, single dose | ~90% (early‑treatment trials) | Injection site reactions, rare allergic events | Conditional EU, Emergency use (US) |
Decision Factors to Weigh
Choosing the right antiviral isn’t just about headline numbers. Below are the practical lenses most clinicians and patients use.
1. Timing of Therapy
All oral options need to start within five days of symptom onset. Paxlovid’s higher efficacy shines when given as early as possible. Molnupiravir stays viable a bit later (up to day7 in some protocols) but loses potency.
2. Drug‑Drug Interactions
Ritonavir, the booster in Paxlovid, is a potent CYP3A4 inhibitor. That means many heart meds (e.g., statins, certain anti‑arrhythmics) require dose adjustments. Molnupiravir’s metabolic pathway is simpler, making it a safer bet for poly‑pharmacy patients.
3. Organ Function
Remdesivir needs decent kidney function (eGFR>30mL/min) and hepatic monitoring. Molnupiravir is contraindicated in pregnancy, while Paxlovid can aggravate severe liver disease due to ritonavir’s metabolism.
4. Administration Convenience
Oral pills win for home isolation - no clinic visits. IV remdesivir or antibody infusions demand infusion centres, which can be a barrier in rural settings.
5. Variant Sensitivity
Monoclonal antibodies lose activity quickly when the spike mutates. Paxlovid and Molnupiravir target conserved viral enzymes, keeping them effective across most variants reported up to 2025.
Best‑Fit Scenarios
- High‑risk patient on multiple meds, early infection: Paxlovid, after reviewing drug interactions.
- Pregnant woman or breastfeeding mother: Use monoclonal antibodies (if available) - molnupiravir is avoided.
- Patient with severe kidney disease unable to take oral meds: Remdesivir, administered in a supervised setting.
- Limited access to pharmacy‑picked drugs, but pills are stocked: Molnupiravir offers the simplest logistics.
Potential Pitfalls & How to Avoid Them
Missing the treatment window - most antivirals stop working after day5. Encourage rapid test‑to‑treatment pathways, like tele‑health scripts within 24hours of a positive result.
Ignoring drug interaction alerts - electronic health records should flag ritonavir‑related cautions. If a patient is on a contraindicated statin, switch to a low‑dose alternative or pause the statin during treatment.
Assuming all patients can tolerate oral pills - nausea or swallowing issues may necessitate IV options. Offer anti‑emetics or consider a short course of remdesivir.
Future Outlook
The antiviral arena continues to evolve. New oral protease inhibitors (e.g., ensitrelvir) are in late‑stage trials and promise similar efficacy to Paxlovid with fewer interactions. Combination regimens - pairing a protease inhibitor with a mutagenic agent like Molnupiravir - are being evaluated to blunt resistance.
For now, the hierarchy remains clear: Paxlovid delivers the strongest protection, Molnupiravir provides a convenient fallback when interactions are a concern, Remdesivir is the IV workhorse for early hospitalization, and monoclonal antibodies serve as a high‑efficacy, variant‑specific bridge.
Frequently Asked Questions
Can I take Molnupiravir if I’m pregnant?
No. Molnupiravir is classified as potentially mutagenic and is contraindicated during pregnancy. Health‑care providers should use alternatives such as monoclonal antibodies or, if available, Paxlovid with careful monitoring.
How do I know which antiviral is covered by my insurance?
Coverage varies by plan and country. In the UK, the NHS generally supplies Paxlovid and remdesivir for eligible high‑risk patients; Molnupiravir may be prescribed where Paxlovid is contraindicated. Always check the formulary or ask your pharmacist.
Do I need a lab test before starting an antiviral?
A rapid antigen or PCR test confirming COVID‑19 is required. For Paxlovid, clinicians also assess kidney function (creatinine) and review current medications. No special labs are needed for Molnupiravir unless pregnancy is a concern.
Can I combine Molnupiravir with Paxlovid for extra protection?
Current guidelines advise against combining antivirals unless part of a clinical trial. The risk of overlapping toxicities and unclear pharmacodynamics outweighs any theoretical benefit.
What should I do if I miss a dose of Molnupiravir?
Take the missed dose as soon as you remember, then continue with the next scheduled dose. If it’s almost time for the next dose, skip the missed one - don’t double‑dose.




Comments
Carlos A Colón
Wow, another pill to juggle, right? If you’re already swamped with meds, just love that Molnupiravir doesn’t need a pharmacy‑run booster, but hey, your liver probably threw a party when you saw the list of contraindications. It’s like the friend who shows up uninvited with a bottle of wine you can’t drink. Sure, the 30% reduction sounds nice, especially when compared to that 89% miracle‑cure that comes with a side of drug‑drug chaos. In the end, pick whatever keeps you out of the ER without turning your med list into a game of Tetris.
Aurora Morealis
Molnupiravir is oral. No IV needed. Good for people who can’t get to a clinic. Efficacy is lower than Paxlovid. Still better than nothing.
Sara Blanchard
Let’s remember that access to antivirals isn’t the same everywhere, and many patients feel left out of the conversation. Molnupiravir’s shelf‑stable pills can be a lifeline for rural communities where infusion centers are miles away. While Paxlovid offers higher efficacy, its drug‑interaction profile can be a barrier for folks on multiple prescriptions. For pregnant patients, monoclonal antibodies remain the safest choice. It’s crucial that clinicians discuss all options openly, respecting each person’s circumstances and cultural background. Ultimately, the right drug is the one that a patient can actually take safely and consistently.
Anthony Palmowski
Are you kidding me??!! This ‘nice‑talk’ about pills while ignoring the elephant in the room-Paxlovid’s 89% isn’t a myth, it’s the real deal!!! If you think a mediocre 30% drop is enough, you’re living in la‑la land!!! Doctors need to stop sugar‑coating and start prescribing what actually works!!!
Jillian Rooney
I cant stand how everyone pretends every drug is the same. Molnupiravir is for the weak, not for real Americans who demand the best. If you'r not takin the top tier like Paxlovid, u r just settlin for second class care. Our nation deserves the strongest medics, not some half‑baked pill!!
Candace Jones
While it’s true that Paxlovid shows higher efficacy, it isn’t suitable for everyone due to its interaction profile. Molnupiravir can still play a valuable role for patients who can’t use Paxlovid safely. Clinicians should weigh the benefits against the risks on a case‑by‑case basis. It’s important to keep the conversation evidence‑based rather than nationalist rhetoric.
Robert Ortega
It’s easy to get caught up in the hype around the newest antivirals, but the reality is that each option fills a different niche. Oral pills like Molnupiravir simplify logistics, especially for home isolation. IV treatments and monoclonal antibodies serve those who need higher potency or have contraindications. Keeping an open mind helps us match the right therapy to the right patient.
Elizabeth Nisbet
Exactly, and adding a quick decision‑tree to your practice can save time. Start with timing – within five days? Then check kidney function and current meds. If there’s no major interaction, Paxlovid wins; otherwise, consider Molnupiravir or a monoclonal antibody if available. This kind of streamlined approach keeps patients safe and reduces confusion.
Sydney Tammarine
Oh my gosh, the drama of trying to choose a COVID pill is literally the plot of my life right now 😂💅. I mean, what if the virus mutates again and all these stats become ancient history? It’s like watching a soap opera where the hero is a tiny capsule and the villain is… pharmacy stock shortages! 🙃
josue rosa
When evaluating oral antivirals for outpatient COVID‑19 management, it is essential to integrate pharmacokinetic data with real‑world effectiveness metrics. Molnupiravir exhibits a rapid absorption profile with peak plasma concentrations achieved within one to two hours after dosing. Its active metabolite, NHC, distributes broadly across tissues, enabling viral RNA incorporation during replication cycles. The drug’s mechanism-inducing lethal mutagenesis-has the theoretical advantage of maintaining activity across divergent SARS‑CoV‑2 variants. Clinical trial data, however, reveal a modest 30 % relative risk reduction in hospitalization compared with placebo, which translates to a number needed to treat of approximately 50 for high‑risk populations. In contrast, Paxlovid’s protease inhibition yields an 89 % reduction, underscoring a substantially greater therapeutic index when drug‑interaction considerations can be managed. Renal clearance of Molnupiravir is minimal, allowing use in patients with eGFR down to 30 mL/min, but the drug is contraindicated in pregnancy due to mutagenic concerns demonstrated in animal models. For patients on polypharmacy regimens, the absence of ritonavir in Molnupiravir simplifies management, yet clinicians must remain vigilant for potential additive gastrointestinal effects when co‑prescribed with other nausea‑inducing agents. Adherence is a pivotal factor; the twice‑daily dosing schedule over five days can be compromised by side effects such as mild nausea or dizziness, which were reported in roughly 10 % of trial participants. Moreover, the timing of initiation strongly influences outcomes, as antiviral efficacy diminishes markedly after day five of symptom onset. Health systems should therefore prioritize rapid testing and prescription workflows to capture the therapeutic window. From a public‑health perspective, Molnupiravir’s shelf stability and lack of cold‑chain requirements make it an attractive option for distribution in resource‑limited settings. Nevertheless, cost considerations and insurance coverage variability can impede access, necessitating advocacy for equitable formulary inclusion. Future research into combination regimens, such as pairing a protease inhibitor with a mutagenic agent, may enhance viral suppression while mitigating resistance development. In summary, Molnupiravir occupies a niche where convenience outweighs maximal efficacy, and its appropriate use hinges on individualized risk‑benefit assessment.
Soumen Bhowmic
I’ve been working with community clinics on COVID‑19 treatment pathways, and we found that tailoring the antiviral choice to the patient’s logistical reality makes a huge difference. For example, in a mobile health van in Delhi we stocked Molnupiravir because it doesn’t need refrigeration, and the patients appreciated the take‑home pills. When a high‑risk patient on statins came in, we switched to Paxlovid after a quick drug‑interaction check, which saved a potential hospitalization. The key is having a simple decision‑tree that considers timing, renal function, and medication burden. We also train the staff to flag pregnancy early so the alternative monoclonal antibodies can be arranged. Overall, flexibility in the formulary lets us adapt to each case without delay.
Jenna Michel
Great points! I totally agree-having that quick algorithm is essential, especially when you’re juggling eGFR thresholds, drug‑interaction matrices, and supply chain hiccups. Let’s make sure we also embed a reminder for tele‑health follow‑up; that way patients stay on track, and providers can catch any adverse events early. By the way, the cost‑effectiveness analysis you mentioned could be bolstered with real‑world data from the electronic health record-just a thought! Keep up the awesome work, and feel free to ping me if you need any extra data or help setting up the decision support tool.
Abby Richards
Molnupiravir works fine for most people 😊
Lauren Taylor
When we talk about equitable access to antivirals, it’s important to frame the discussion within the broader context of health disparities that have been magnified throughout the pandemic. Molnupiravir, with its temperature‑stable formulation, can serve as a critical bridge in communities that lack infusion centers or reliable cold‑chain logistics. However, the 30 % efficacy must be communicated transparently so patients can make informed decisions alongside their clinicians. Integrating culturally competent counseling into prescribing practices can mitigate mistrust and improve adherence, especially among racial and ethnic minority groups. Moreover, pharmacy outreach programs that deliver the medication directly to homes can reduce barriers related to transportation and work schedules. By aligning public‑health policy with these practical implementation strategies, we can ensure that the benefits of oral antivirals are not confined to privileged subsets of the population. Ultimately, the goal is a harmonized approach that leverages both high‑efficacy options like Paxlovid and pragmatic solutions like Molnupiravir to close the treatment gap.
Vanessa Guimarães
It is frankly astonishing how some individuals continue to champion sub‑optimal therapies while ignoring the overwhelming evidence favoring protease inhibitors, a phenomenon that would make a conspiracy theorist blush. One might suspect that the promotion of Molnupiravir is less about scientific merit and more about a desperate quest for nationalistic bragging rights, as if our pharmaceutical sovereignty depends on a drug that barely nudges hospitalization rates. The data are transparent: an ~30 % reduction versus ~89 % for the gold standard, a disparity that should settle the debate once and for all. Yet, the discourse persists, fueled by misinformation and an odd reverence for “any” FDA‑authorized product. In a world where we ought to prioritize patient outcomes above political posturing, clinging to a mediocre antiviral is simply indefensible.