From O’Dwyer’s PR Blog:
The Vytorin controversy is just icing on a (low cholesterol) cake for those upset with the barrage of drug advertising, and the promotion of “quasi diseases” by big pharmaceutical houses. It’s a safe bet the Congress is going to step up efforts to regulate the marketing practices of prescription drugs.
Bart Stupak, chair of the Energy and Commerce investigations subcommittee told the WSJ that is sick and tired on seeing “puffing, advertising based on untrue facts or facts that can’t be substantiated, medical, ethically or legally.” Ouch.
Even the Rx industry’s trade group, Pharmaceutical Research and Manufacturers of America, is raising the white flag. Chief Billy Tauzin admits that some of the criticism of the industry is valid and needs to be addressed. He promises that the industry will consider major changes.
That’s a major concession from the once all-powerful PhRMA, but one that is grounded in the reality of the various pharmaceutical investigations on Capitol Hill and the prospects of a Democrat in the White House.
Oh what fun it is to cover Big Pharma. First we get the Wall Street Journal crediting Peter Rost and BrandweekNRX for breaking an insider trading scandal at Schering-Plough.
Then we get Merck and Schering-Plough pulling their popular “two sources of cholesterol” commercials from television because Vytorin apparently does not reduce the buildup of fatty plaque as claimed.
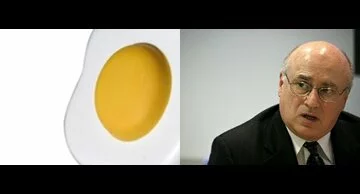
As Media Orchard puts it, there are
Two Ways to Get Egg on Your Face
1. You can crack one open.
2. You can be Merck CEO Richard Clark.
Double whammy for Big Pharma in Advertising Age today. First, a story headlined “Vytorin Ad Shame Taints Entire Marketing Industry” — an excerpt:
Reports that [as] Merck & Co. and Schering-Plough Corp. kept under wraps for more than a year findings that Vytorin does not deliver results it spent more than $100 million advertising to consumers is much more than a PR disaster for the drug’s co-marketers.
Coming on the heels of a New York Times story that Pfizer’s $2 billion drug Lyrica treats a condition, fibromyalgia, that a lot of doctors don’t think exists, the Vytorin news is fanning the flames of public mistrust for the $5 billion direct-to-consumer drug industry — and the ad business in general.
“The pharmas are in big trouble in terms of credibility,” said brand expert Rob Frankel, who runs his own consultancy at RobFrankel.com. “They’re just above Congress and used-car salesmen.”
Accompanying this story is an editorial headlined, “Drug Companies One Step Away From More Regulation.” In it, Ad Age warns:
If drug companies aren’t more careful, they’re going to come down with a case of regulatory pneumonia and the bad-publicity flu.
We’re firm believers that a company selling legal and safe products should be allowed to advertise them. But as is often the case, an ounce of prevention goes a long way. In particular, drug marketers would be wise to tread carefully when rushing the next blockbuster not only to market but onto the airwaves.
We understand that research isn’t cheap. And a blockbuster such a Viagra or a Lipitor goes a long way in paying for life-saving drugs. But what’s much more expensive than research and marketing is fighting lawsuits on numerous fronts or being silenced across the board by the government…
We know that research doesn’t pay for itself and that drug marketers need to make money, but those thinking of making the hard sell for new drugs should consider the physician’s motto: “First, do no harm.”
-
 Subscribe in a reader
Subscribe in a reader -
Search Blog Posts
-
-
-
How to Safely Buy Prescription Drugs Online from Cary Byrd on Vimeo.
-
Archives
-
Our Healthcare100 Ranking