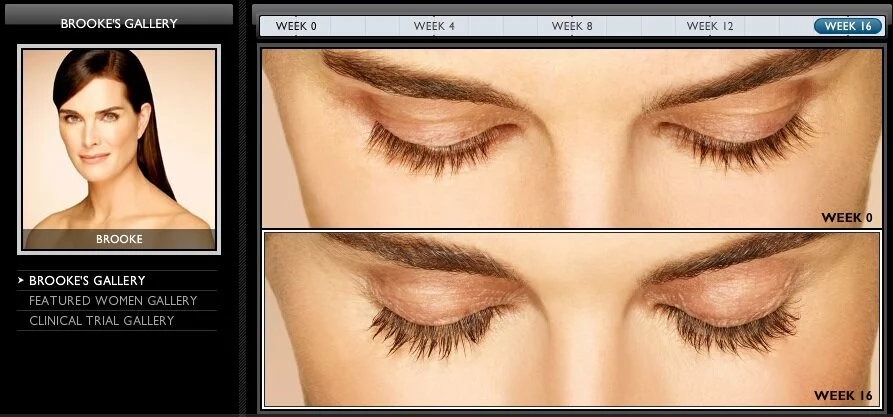
Some people have very sparse eyelashes, and this can cause functional problems with the eye as well as anxiety about one’s appearance. Fortunately for people suffering from ” eyelash hypotrichosis,” a brand-new treatment was approved by the FDA in December 2008. The product is called Latisse, it has sultry-eyed Brooke Shields as a spokesmodel, and it is a 0.03% solution of bimatoprost marketed with an applicator brush.
Latisse is meant to be dabbed along the base of the upper lash line (the lower is too sensitive), causing hair there to grow in much more thickly and darkly than before. Its effects are not permanent, and if it is discontinued, the lashes will return to their original condition within weeks or months.
Technically, bimatoprost is a prostaglandin analog that affects eye pressure. As a generic, and under the trade name Lumigan, the solution is used to control the progression of glaucoma and manage ocular hypertension. Although the main ingredient is the same, using bimatoprost or Lumigan, which are serious ocular pressure meds, to lengthen or grow eyelashes is an unapproved, off-label use. (In fact, eyelash growth was a surprise side effect of these meds.) Some are tempted to swap these pharmaceuticals freely, but doctors warn that it can be harmful to your health profile and insurance history to be have a history of ordering glaucoma drugs when you are merely trying to treat a cosmetic problem.
Commercials for Latisse have been in heavy rotation on television and in glossy women’s magazines lately. If you aren’t paying close attention, you might think you are looking at a mascara ad, but mentions of the treatment being the “first and only†treatment approved by the FDA to treat hypotrichosis make it clear that this is not just make-up. The glam may have gone a little too far, though, says the FDA. MSNBC reports:
Last week, Latisse made headlines when the FDA sent a warning letter to Allergan stating the promotional materials posted on the product’s Web site were “misleading because they omit and minimize risks associated with Latisse.†Among the risks, the FDA notes, is that the active ingredient can cause hair to grow in other places besides the lash area, cause inflammation of the cornea — and can make lighter-colored eyes turn brown.
Meanwhile, an article at BNET says the FDA also accused Allergan of downplaying infection risks.
We went directly to the Latisse website to get their own description of the side effects. Here’s a summary: About 4% of users experience itchy and/or red eyes. Less common side effects include skin darkening, eye irritation, dryness of the eyes, and redness of the eyelids. It is very important not to use Latisse directly on the eye, or on the lower eyelid. It should only be applied to the UPPER eyelid with a sterile applicator. Latisse may cause darkening of the eyelid skin which may be reversible, and it may also cause increased brown pigmentation of the colored part of the eye which is likely to be permanent.
The information is there, but not particularly prominent. Changing the color of the eye is a huge side effect, and we’re curious how the public will react. Latisse is a very popular product, getting huge traffic on beauty boards as people compare notes on how to solve their lash problems without makeup. It will be interesting to see what develops as the FDA and Allergan come to an agreement on how to present side-effect information fairly.
-
-
Search Blog Posts
-
Save Even More Money!
-

-
Trending Content
-
Watch our YouTube Video
-
Categories
Big Pharma Buy prescriptions online Canadian drugs Drug costs Drug reimportation Drug safety eDrugSearch.com FDA Health 2.0 Healthcare100 Healthcare blogs Healthcare solutions Low-cost drugs Medicare Part D Merck Online pharmacies Online pharmacy safety Pfizer Pharma bloggers Pharmaceutical companies Pharmaceutical marketing Pharma cheerleaders Prescription drug abuse Prescription drug prices Prescription drugs Prescriptions Wal-Mart drug plan -
Blogroll
- Bullet Wisdom
- Bulverde Business Directory
- Christian Counseling San Antonio Tx.
- Christian Schools in San Antonio Texas
- Christian Social Network
- Christians United for Israel
- DrugWonks.com
- Eye on FDA
- GoozNews
- Health 2.0
- Hunting Forum
- In the Pipeline
- Jesus Christ Our King
- John Hagee Ministries
- Kevin, M.D.
- Local Search Marketing
- My $299 Website
- Pharm Aid
- Pharma Marketing
- PharmaGossip
- Pharmalot
- San Antonio Asphalt
- San Antonio Life Insurance
- San Antonio Pressure Washing
- Storage New Braunfels Tx
- Texas Wildlife Supply
- The Angry Pharmacist
- The Health Care Blog
- The Peter Rost Blog
- World Vision
-
Tags
big pharma Canadian drugs canadian pharmacies canadian pharmacy consumer reports craig newmark divine healing Drug costs drug prices Drug reimportation eDrugSearch.com FDA Fosamax Generic drugs healing scriptures Health 2.0 healthcare reform Hypertension Jehova Rophe Jesus Christ Lipitor Metformin miracles nabp online pharmacy dictionary online prescriptions osteoporosis peter rost Pharmacies pharmacists pharmacychecker pharmacy spam phrma Prescription drugs prescription medication Proverbs 3:5-8 reimportation relenza Roche saving money SSRI swine flu Tamiflu The Great Physician The Lord our Healer -
Recent Tweets
- eDrugSearch Blog Rank on the Healthcare100: http://t.co/VJprL4LZWl [#]
- New blog posting, How to Get Prescription Medication Without Health Insurance - http://t.co/1ZdLavB87d [#]
- 10 Tips for Safer Prescription Drug Use http://t.co/GFnMIN1mCy [#]
- New blog posting, How to Beat High Drug Prices By Comparing Low Cost Pharmacies - http://t.co/fsZ0stNZme [#]
-
Archives
-
Recent Comments
- Heather Sturges on What is the Difference Between Effexor and Cymbalta?
- Lupe Machol on Cost of diabetes drugs has nearly doubled
- Manpower For Hospital In Pune on Why is Medicine Cheaper in Canada?
- Jen on How a Canadian Pharmacy Can Help You Offset Drug Price Hikes
- nino iarajuli on Vending machine dispenses prescription drugs