
Earlier this month, a closely-watched trial over the osteoporosis drug Fosamax ended in mistrial, to the frustration of nearly everyone involved. The trial was marked by great tension, with a deadlocked jury, reports of threats of physical violence, and a judge-ordered cooling-down period.
What could cause such intense drama? Well, this was just one of approximately 900 state and federal cases pending against Fosamax, alleging that that medication causes osteonecrosis of the jaw (the death of jawbone tissue). In large part, the tension in the Manhattan courtroom was that this trial — the first — was supposed to be an indicator of how these hundreds of similar cases might proceed. The other major factor is that it is notoriously difficult to “prove†drug-related injuries, and this difficulty was definitely shown in the frustration and tension among jury members.
Millions of women have taken Fosamax (alendronate), a Merck drug that was approved in 1995 to treat osteoporosis associated with menopause, and in 1997 to prevent osteoporosis itself. Until the recent introduction of some competing medications, it was one of the most popular drugs in the U.S. It is still prescribed millions of times per year to women suffering from bone loss.
In short, Merck’s defense on this topic is that there is no definitive evidence that Fosamax causes the death of jaw tissue, while plaintiffs and their lawyers insist that Merck overpromoted Fosamax without warning doctors about the potential for jaw injury. Obviously, no conclusions were reached.
Of course, this is of great interest to women who may have taken, or are considering taking, Fosamax. Only your doctor can decide what is the right choice for you, but we thought we would enumerate the other medications available for treating osteoporosis for those doing research on Fosamax alternatives. One very important thing to be aware of it is that Fosamax is not the only osteoporosis drug in this class (the bisphosphonates), which all have a similar mode of action. Fosamax is being talked about most in the media, but all are associated with some amount of risk of damaging the jaw.
If you do choose to take a bisphophonate, know these facts:
- A high proportion of jaw injuries occur following high-dose intravenous administration, so that is a particularly risky way to take the medication.
- As many as 60% of the cases are preceded by a dental surgical procedure involving the jaw. In short, women who are expecting major dental work should consider delaying treatment with Fosamax, Boniva, Actonel or similar drugs until after their dental surgery.
Here are the main alternatives on the market for Fosamax:
- Actonel (risedronate) is a bisphosphonate manufactured and marketed by Procter & Gamble and Sanofi-Aventis. It belongs to the same family of drugs as Fosamax, and may be associated with the same jaw side effects.
- Boniva (ibandronate) is also a bisphosphonate, manufactured and marketed by GlaxoSmithKline and Roche Laboratories. It is a competitor to Fosamax, and may also affect the jaw tissue.
- Evista (raloxifene) is NOT a bisphosphonate , but rather an oral selective estrogen receptor modulator from Eli Lilly and Company that affects bones through estrogen. Evista may be an interesting alternative for women concerned about their jaw health or planning oral surgery.
Also this month, The Wall Street Journal published an article called “From the Osteoporosis Front, Updates on Potential New Drugs.†These are the up-and-comers in clinical trials and going before the FDA. The story covers the latest news about Wyeth’s Viviant, Pfizer’s Fablyn, Amgen’s denosumab, and other upcoming treatments for osteoporosis.
If you are interested in following the Fosamax case that ended in a mistrial, it is expected to be re-tried in the spring. The name of the case is “In re Fosamax Products Liability Litigation, U.S. District Court, Southern District of New York (Manhattan), No. 06-1789.”
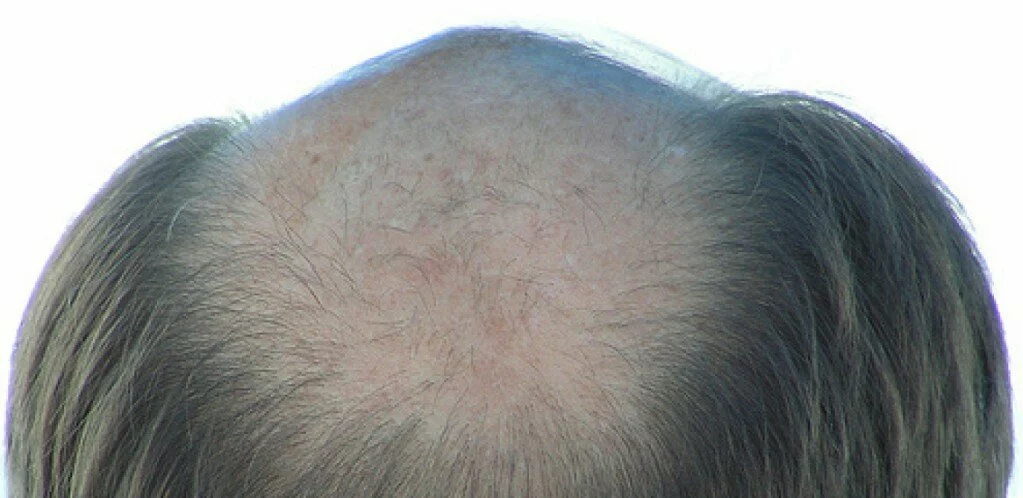
Are you one of millions of people battling some type of hair loss? More than half of males experience some degree of male pattern baldness by age 50, but even women and children can experience unwanted hair loss. There are more options than ever before to treat hair loss — such as herbal treatments, scalp massage, lasers, and surgery — but one of the most popular options is the safe and effective pharmaceuticals on the market.
There are two main medications approved by the FDA for treatment of hair loss — and they’re very different. For starters, Rogaine is a topical solution applied to the scalp, while Propecia is an orally administered pill. One medication is better than the other at treating a receding hairline. Men can use either medication, or even both, but women are restricted to just one. Because of these distinctions, it is important to choose the right hair-loss medication for you.
Let’s take a closer look at each one.
Rogaine (minoxidil) was first on the scene. Its ability to fight hair loss was discovered accidentally. The drug, a vasodilator, was originally used exclusively to treat high blood pressure, when some patients began reporting that it re-grew hair as a side effect. It was approved by the FDA in 1988 to treat male pattern baldness. Rogaine, by Pfizer, is primarily effective at stopping hair loss, but in some patients, it can increase protein blocks, which can promote new hair growth. It is said to be effective both on the hairline and vertex of scalp. Rogaine can be used by both men and women, in a 2% or 5% solution.
Propecia (finasteride), made by Merck, is an orally administered medication approved by the FDA in 1997 to treat hair loss. Unlike Rogaine, which is a vasodilator, Propecia acts on hair loss through hormonal means. (Because of this, Propecia should NOT be used by women or children; it could be very dangerous.) Propecia is an anti-androgen, which decreases the conversion of testosterone to dihydrotestosterone (DHT), a chemical responsible for balding. With DHT inhibited, existing hair is better maintained, and the body can put more energy into thinning follicles so that they become thicker. Propecia has high effectiveness with early to moderate hair loss, and works best on the crown of the head, but not as well with a receding hairline.
Neither Rogaine nor Propecia is a quick fix for hair loss. They both need to be taken for long periods. It can take anywhere from 6 to 24 months to see initial results, and patients may need to take their chosen medication indefinitely keep treating the condition.
Knowing all this, which factors should guide your choice between Rogaine and Propecia?
- Your gender: if you are male, you can be prescribed either (or both), but if you are a woman, you can only use Rogaine.
- Your goals: Rogaine is slightly more useful for retaining existing hair, while Propecia is said to be more effective at promoting new hair growth.
- Your area of hair loss: Propecia has good results mainly on the crown, while Rogaine has documented success on the hairline and the crown.
- Medical interactions: Consult your physician to determine which drug is a better fit for your personal health conditions and other medications.
- Side effects: With Propecia, you may experience decreased libido or gynecomastia. Rogaine may trigger allergic effects, chest pain, dizziness, or irregular heartbeat.
- Ease of use: Some people feel that a daily pill is simpler than a twice-daily application to the scalp, but others prefer to pick their medication based on its mode of action.
Whichever method you select, be patient, and remember to keep feeling good about yourself, your hair, and your overall health. As with any drug, please consult your physician before you begin any medication.
A diagnosis of high cholesterol can be intimidating, but there is a lot you can do to control this condition. In addition to modifying your diet and upping your exercise, the addition of a HMG-CoA Reductase Inhibitor — a class of drugs commonly called “statins†— can safely and effectively lower your cholesterol. (HMG-CoA Reductase helps our liver produce cholesterol; when the chemical is inhibited, the amount of cholesterol is correspondingly reduced.) For people with heart disease, statins can lower the risk of a cardiac event and subsequent death. If you and your doctor have determined that you need a statin, how can you pick the right statin for your needs?
There are six statins on the market: atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin. They differ in their ability to reduce cholesterol, and they also differ in their rates of reducing heart attacks. Their costs are also quite different — and since most people take statins for a long time, the costs add up over the years. With all of these variables, choosing the right statin for you can be complex.
All statins are capable of lowering LDL (“badâ€) cholesterol and triglycerides, and raising HDL (“goodâ€) cholesterol. The statins do differ in how effectively they can do this, and it is highly dose-dependent. Says Drug Digest:
If the needed LDL-C reduction is up to 35-36%, any of the statins should be acceptable choices for therapy. For a desired reduction of LDL-C greater than 42%, simvastatin (Zocor), atorvastatin (Lipitor), or rosuvastatin (Crestor) would be needed.
Indeed, the best-known statins are Crestor, Lipitor, and Zocor (quite probably because they have the greatest effect on cholesterol levels). The latter two are also endorsed by Consumer Reports. Taking evidence for effectiveness, safety, and cost into account, the publication rated both of these statins as “Consumer Reports Best Buy Drugs.†They recommend:
• Generic simvastatin (20mg or 40 mg) — if you need 30% or greater LDL reduction and/or have heart disease or diabetes, or if you have had a heart attack or have acute coronary syndrome and your LDL level is not highly elevated.
• Atorvastatin (Lipitor) (40mg or 80mg) — if you have had a heart attack or have acute coronary syndrome and your LDL is highly elevated; use for two years and then reconfirm need or switch to generic simvastatin.
Charts on Drug Digest have some great comparisons. For instance, they show that Lipitor (10-80 mg.) can reduce total cholesterol by 25-45%, while Zocor (5-80 mg.) can reduce the same numbers by 19-36%, and Crestor (5-40 mg.) can reduce it by 33-46%. As for lowering HDL, Lipitor can offer reduction of 5-9%, Zocor lessens HDL by 8-16%, and Crestor lowers these numbers by 8-14%. As you can see, choosing the proper statin has a lot to do with which numbers (Total Cholesterol, HDL, LDL, or triglycerides) you are trying to effect.
A final consideration is that last year there was reporting on an observational study done by Pfizer that suggested that there were certain benefits to using Lipitor over Crestor. However, one must keep in mind that Pfizer conducted the study, and they are the manufacturer of Lipitor, and they are defending this drug against Merck’s Zocor product, which is now available in a generic formula. Here is the information as presented by The Wall Street Journal:
An analysis, published in the latest Clinical Therapeutics Journal, mined a large database of health-care records and found that patients taking Lipitor had a 12% lower risk of a cardiovascular event than those on simvastatin, the generic name for Zocor. The patients on Lipitor had a 15% lower risk of having a heart attack.
So-called observational studies like this one that look at data after the fact aren’t as powerful as prospective clinical trials. Jack Tu, a cardiologist who specializes in outcomes research at Canada’s Institute for Clinical Evaluative Sciences, says the latest Pfizer study didn’t take into account factors that could predispose a patient to heart problems, such as smoking and cholesterol levels. “Just on this alone, you wouldn’t recommend that everyone should switch onto Lipitor,†he says.
Still, Pfizer hopes that doctors will take notice. “We’ve done two rather large observational studies and patients have a lower risk of cardiovascular events on Lipitor [compared with] simvastatin,†says Susan Shiff, Pfizer’s team leader for cardiovascular outcomes. “Doctors need to factor this into discussions with patients.â€
You should definitely discuss with your physician which statin is right for you. In general, the best plan is to take the LOWEST dose of a statin that gets you to your target level for cholesterol. Overly large doses can be harmful to your liver and to your muscles. If you experience muscle aches and pains when taking a statin, contact your doctor immediately.
Oh what fun it is to cover Big Pharma. First we get the Wall Street Journal crediting Peter Rost and BrandweekNRX for breaking an insider trading scandal at Schering-Plough.
Then we get Merck and Schering-Plough pulling their popular “two sources of cholesterol” commercials from television because Vytorin apparently does not reduce the buildup of fatty plaque as claimed.
As Media Orchard puts it, there are
Two Ways to Get Egg on Your Face
1. You can crack one open.
2. You can be Merck CEO Richard Clark.
From the Daily Dog:
The state and city of New York filed suit this week against Merck, claiming the drugmaker concealed the risks of the withdrawn arthritis treatment Vioxx. The lawsuit seeks damages, penalties and restitution for “tens of millions of taxpayer dollars wrongfully spent on Vioxx prescriptions,” the office of New York State Attorney General Andrew Cuomo said in a statement… “Merck’s irresponsible and duplicitous conduct endangered the health of New Yorkers and wasted our tax dollars,” Cuomo said…
The Attorney General’s office said this is the first case to be brought under New York’s recently-enacted False Claims Act, which allows the state to seek damages for the amount spent in Medicaid and EPIC healthcare programs to pay for drugs prescribed under false pretenses … Between 1999 and 2004 Medicaid and EPIC spent over $100 million on Vioxx prescriptions in New York State, the Attorney General’s office said, referring to the New York State Medical Assistance Program and the Elderly Pharmaceutical Insurance Coverage plan.
Remember the little problem with Vioxx? As a result, Vioxx still has more than 13,800 lawsuits on the docket. Over the last two years, only four trials have been held, but Judge Carol Higbee is looking to pick up the pace — from glacier slow to snail-in-winter slow. Higbee’s goal, according to Pharmalot, is to “ease the logjam”. But Pharmalot points out:
With so many lawsuits, it’s entirely possible that many of the Vioxx plaintiffs will never see their day in court, or collect if they do win, because Merck is appealing every outcome that doesn’t go its way. For this reason, Wall Street believes Merck’s Vioxx liability is $5 billion, not the $25 billion or more that many once estimated.
Lesson for Big Pharma: Sometimes it’s better for your product to suck a lot, rather than just a little. (Especially for your lawyers.)
From Reuters:
Brazil’s president is to decide whether his country will honor Merck & Co’s AIDS drug patent, after the health ministry rejected the company’s price-cut. “We consider the offer insufficient and we told the manufacturer,” Brazil’s health minister, Jose Temporao, told Reuters on Thursday. “The decision (on whether to break the patent) is now being analyzed by the president.”
The government said last week it was considering importing generic versions of the drug, Efavirenz, for Brazil’s lauded AIDS treatment program if it decides not to honor the patent. It has not mentioned any plans to make the drug locally. Brazil has threatened to break patents before, but has hammered out deals in the end. Under World Trade Organization rules, a country can sidestep patents by issuing a “compulsory license,” which allows production and imports of generic drugs for public health and national emergencies. Brazil declared the drug “in the public interest” and too expensive to buy.
Brazil wanted Merck to cut the price of Efavirenz to $0.65 per pill — the same price paid by Thailand — from $1.57 per pill paid by Brazil, the ministry said. A source close to the negotiations said the New Jersey-based drugmaker has since come back with an offer of $1.10 a patient per day, but Brazil rebuffed that bid. The source said the talks were at an impasse.
Can you imagine the U.S. government standing up to drug companies in this way? Unfortunately, it would never happen.
Besides the unexpected public outcry, there may have been another reason why Merck curtailed its lobbying efforts to make Gardasil mandatory for teens.
The stuff may not even work very well.
From GoozNews:
The Wall Street Journal ran a front-page story today questioning the efficacy of Merck’s new cervical cancer vaccine. The story covers all the key issues: the relative rarity of the disease; how it’s been reduced by 80 percent in the U.S. through Pap smears; and the relatively low 70 percent effectiveness rate of the new vaccine. One key detail new to me: Merck cancelled its lobbying push in the states after consulting with key members of the Centers for Disease Control advisory committee and the American Academy of Pediatrics that had recommended the vaccine. They told the company that it was too soon to make the vaccine mandatory.
The story was also notable in pointing out that all the cost-effectiveness studies arguing that the vaccine would reduce health system costs (fewer Pap tests and fewer cancers down the road versus the multi-billion upfront costs of the vaccine) were funded by the companies that made the vaccine.
Yesterday, an FDA panel voted 20 to 1 not to approve Arcoxia for osteoarthritis. Why such an overwhelming number?
Apparently, Arcoxia failed to prove that it served an unmet need. According to Merrill Goozner, Merck took some pretty poor evidence with them to the trial.
Merck stepped forward with an extremely weak set of data. They tested the drug in a staggering 34,000-plus patients, half of whom took diclofenac (sold as Voltaren or Cataflam), which has very low market penetration in the U.S. What it does have is a heart attack risk profile that is very similar to other Cox-2 inhibitors.
“What is the value of comparing it to diclofenac, which has an elevated cardiovascular risk?” asked [FDA safety officer David] Graham near the conclusion of his devastating critique. “It has no value.”
Ouch.
-
Search Blog Posts
-
-
Trending Content
-

-
Blogroll
- Bullet Wisdom
- Christian Social Network
- DrugWonks.com
- Eye on FDA
- GoozNews
- Health 2.0
- In the Pipeline
- Jesus Christ Our King
- Kevin, M.D.
- Pharm Aid
- Pharma Marketing
- PharmaGossip
- Pharmalot
- San Antonio Asphalt
- San Antonio Life Insurance
- San Antonio Pressure Washing
- The Angry Pharmacist
- The Health Care Blog
- The Peter Rost Blog
- World Vision
-
Tags
big pharma Canadian drugs canadian pharmacies canadian pharmacy consumer reports craig newmark divine healing Drug costs drug prices Drug reimportation eDrugSearch.com FDA Fosamax Generic drugs healing scriptures Health 2.0 healthcare reform Hypertension Jehova Rophe Jesus Christ Lipitor Metformin miracles nabp online pharmacy dictionary online prescriptions osteoporosis peter rost Pharmacies pharmacists pharmacychecker pharmacy spam phrma Prescription drugs prescription medication Proverbs 3:5-8 reimportation relenza Roche saving money SSRI swine flu Tamiflu The Great Physician The Lord our Healer -
Archives
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- August 2012
- July 2012
- June 2012
- April 2012
- March 2012
- February 2012
- January 2012
- November 2011
- June 2011
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- January 2010
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- August 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- May 2007
- April 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
-
Recent Comments
- Ranee on What is the Difference Between Effexor and Cymbalta?
- bAnn805 on Crestor, Lipitor, or Zocor – Which statin is right for you?
- Anna Pham on Why is Medicine Cheaper in Canada?
- Anna Pham on How to Get the Cheapest Prescription Medications
- Anna Pham on How to Travel With Prescription Medication
