Today in London the FDA decided to postpone approval of a new experimental heart drug, seeking more information about the new product.
The drug called Certriad, combines AstraZeneca’s blockbuster cholesterol pill Crestor with Abbott Laboratories TriLipix.
According to Rueters,
The manufacturers said on Tuesday they had received a so-called “complete response letter” from the U.S. Food and Drug Administration for Certriad, which combines Astra’s blockbuster cholesterol pill Crestor and Abbott’s TriLipix.
Both companies said they were evaluating the letter from the agency and would respond to the request for additional information. An AstraZeneca spokesman declined to give further details.
Combo pills are fairly common for heart drugs, and most thought that FDA approval was a given. Merck merged Zocor and Zetia to form Vytorin, and soon they plan to combine Zetia with Pfizer’s Lipitor.
Combo pills are also fairly common in diabetes drugs. For example Januvia, Avandia,and Actos are all available as combo product with generic versions Glucophage.
In typical big pharma fashion, Astra did not disclose what the FDA has a problem with, but many analysts have voiced concern that Certraid does not have enough backing to pass FDA approval after the results from a clinical study this month showed there was no real benefit from adding TriLipix to Crestor.
New studies released today found that many who take the popular diabetes medication metformin find the odor off-putting, and some have trouble taking the medication.
The “fishy” odor is especially noticeable in the immediate release versions.
“Metformin is an excellent drug, but the immediate-release formulation may have an odor to it. The smell is fishy or like the inside of an inner tube, and in a patient’s mind, because it smells like something that has gone bad, they may think the drug isn’t good,” explained one of the letter’s authors, J. Russell May, a clinical professor at the University of Georgia College of Pharmacy at the Medical College of Georgia.
However, May said, “some metformin products on the market are extended-release and the drug is embedded and released slow, over time. These products have much less smell, if any.”
May and his colleagues wrote the letter to the journal to raise awareness of this issue, especially because nausea is a commonly reported side effect of metformin. “Is it nausea from the medication, or is it because it smells bad?” May said.
The drug’s odor may make it seem like it has gone bad, but doctors have reassured patients that it is just something in the formula of the medication, and the drug is still affective at treating diabetes and is not dangerous.
Bristol-Meyers Squib who is one of the major producers of metformin released a statement saying,
Bristol-Myers Squibb is aware that the inherent characteristics of metformin have been associated with a mild odor upon opening of the bottle, so these type of reports are not unexpected. It’s important to note there has been no correlation between an odor and the efficacy of metformin, which has been on the market in the U.S. since 1995.
Some of the brand versions of metformin that may give off this “fishy” odor are: Glucophage, Glumetza, Fortamet, and Riomet.
Patients prescribed metformin should continue on their regular regiment, but should certainly let their doctor know if they are affected by the smell. They may be able to switch to the extended release version or possibly another brand.
According to reports, the axe is coming down all over the pharma world on research and development projects that are not yielding immediate results.
AstraZeneca(Atacand, Crestor), GlaxoSmithKline (Advair, Boniva) and Pfizer (Benadryl, Lipitor) have all already begun to scrap projects, while others like Sanofi-Aventis (Allegra, Plavix) are about to pick up the trend and start making cuts.
The cuts come as no surprise, as big pharma companies have been seeing there pipelines shrink since 1998, when the trend to buy out drug rights from smaller bio-tech companies began.
Despite the increased cost efficiency of buying drugs from smaller bio-techs, I am not so sure that big pharma is going to like the end result of their decision.
Stephen Foley raises some excellent questions in a recent post, saying
those calculations about the benefits of in-licensing over in-house could change rapidly if the competition for licensing deals, which has been getting more ferocious for several years, increases dramatically. It could be that they will regret swinging cuts to their R&D budgets sooner rather than later.
And there is another reason for executives to pause. There are very great political benefits from drug companies being able to trumpet the life-changing discoveries that have emerged from their research labs and their scientific trials. Yes, these are companies that have manipulated the publication of scientific data, made over-reaching claims for their drugs, and practiced price gouging of government health and insurance services, but they are also companies that lower our cholesterol, shrink tumors, keep diabetes in check and lift the burdens of depression. In the UK, there is an explicit compact with the government on this score: drug prices charged to the National Health Service are set to allow for investment in research. In the US, the good works of drug research help keep in check the demands for re-importation of drugs from lower-priced Canada, and other cost-cutting measures.
It sounds like big pharma is trying to have their cake and eat it too; outsourcing research and development to cut costs while still maintaining control over patents on drugs to protect their profits.
Cutting the cost of research and development is like cutting off your leg to lose weight. Why not cut the fat of advertisement out first. After all, aren’t doctors suppose to tell us the medicines we need?
After they get rid of the cost of research and development, what excuse will big pharma have left to overcharge consumers?
 Healthcare reform isn’t just about the public option and paying for doctor’s visits — it’s also about equal, affordable access to life-saving medications for all Americans. That’s why many Big Pharma watchdogs are so disappointed with a recent amendment slipped into healthcare legislation that proposes extending patent protection on biologic drugs, delaying for years the public’s access to affordable follow-on versions.
Healthcare reform isn’t just about the public option and paying for doctor’s visits — it’s also about equal, affordable access to life-saving medications for all Americans. That’s why many Big Pharma watchdogs are so disappointed with a recent amendment slipped into healthcare legislation that proposes extending patent protection on biologic drugs, delaying for years the public’s access to affordable follow-on versions.
What are “biologics� They’re the next big wave in medicine — drugs made not from simple chemical formulations, but from biological components. They’re very expensive, and poised for enormous success:
By 2014, the biggest-selling meds will be biologics, according to an analysis from Evaluate Pharma. Taking the place of Pfizer’s gargantuan drug Lipitor will be Roche’s Avastin, a cancer med expected to account for $9.23 billion in 2014 sales. (Even when you factor in the recent trial disappointments.) The next five top sellers, in order, are expected to be Humira (Abbott Labs), Rituxan (Roche), Enbrel (Wyeth/Amgen), Lantus (Sanofi-Aventis), and Herceptin (also Roche).
Evaluate also predicts that half of the top 100 drugs in 2014 will be biotech meds — a huge change from last year’s level of 28 percent and 11 percent in 2000.
Because biologics are so complex, the system we all know — where patented brand names enjoy a period of exclusivity, then eventually make way to cheaper generics — doesn’t translate perfectly. Biologic “generics†are called “biosimilars,†and they are not seen as generic equivalents. They must be submitted for approvals as new drugs and do their own clinical trials, etc.Â
The Eshoo-Barton amendment, named for sponsoring Representatives Anna Eshoo (D – Calif.) and Joe Barton (R – Texas), would give brand-name biologic drugmakers 12 years of market exclusivity. By comparison, President Obama favors seven years, and Rep. Henry Waxman (D –Calif.) feels that the public should have access to “generic†biologics after just five years. By contrast, says Medical News Today, “The Biotechnology Industry Organization maintains that there should be a minimum 14 years of exclusivity to account for a development process that on average takes 10 years and $1.2 billion for a product to reach market.â€
5, 7, 12, or 14 years? As you can see, there is a real difference of opinion on this subject. One person who has written extensively on this is author James Love on the Huffington Post. Here he explains why this amendment is harmful:
The Eshoo/Barton amendment, which has the support of many newly pro-PhRMA democrats, will extend the period of monopolies for biologic medicines, when compared to the original Waxman text. The only question is how long. Part of the harm will be the longer period prohibiting generic suppliers from relying upon evidence that medicines are safe and provide therapeutic benefits. Much of the other harm will come from a number of technical changes in the bill that make it much easier for incumbent firms to block entry through technical issues, extended litigation, and ever-greening of protection from small medically unimportant changes in protected medicines.
This is essentially a case of innovation versus access. Drug companies want protection from the risks and costs borne in the creation and testing of new drugs; patient advocates say that Big Pharma (or Big Biotech, if you like) already make large profits and that the public deserves access to affordable biosimilars in a more timely fashion. “Entities that support longer periods of exclusivity — such as universities, biotech companies and venture capitalists — are ‘fighting to protect inventors’ rights and ensure more thorough clinical trials.’ On the other side, consumer groups, labor unions, insurers and generic drug manufacturers ‘see shorter exclusivity as the way to deliver safe, affordable and quality drugs to patients and open the marketplace to increased competition,’†explains Medical News Today.
The latest high-emotion development is blogger Jane Hamsher’s “Are You Or Someone You Know Paying $50,000 A Year For Drugs?†It paints an ugly picture of what happens to people who cannot affording life-saving biologics. A few days later, Rep. Eshoo responded to this and other online attention with a blog post on The Hill’s Congress Blog titled “Setting the record straight on our health care legislation.†If you check in with these two articles, you’ll have the latest from both side of the “biologic generics” debate.
Our mission, as always, at EDrugSearch.com is to improve the American public’s access to safe, quality medications at an affordable cost.
For more information:
- Should We Be OK With The PhRMA Deal With White House?
- US House Panel Backs Exclusivity for Biologic Drugs
- Pay or die: Deadly Pharma amendment in HCR going right under radar
A new blood-pressure treatment has come on the market that is of potential interest to hypertension patients whose blood pressure is not being adequately controlled with monotherapy.
Last month, the US Food and Drug Administration (FDA) approved Novartis’ Valturna (aliskiren and valsartan), which combines in a single pill valsartan, the active ingredient in Diovan — the top-selling branded high blood pressure medicine — and aliskiren, the active ingredient in Tekturna — the only approved direct renin inhibitor (DRI).
Valturna is the first and only medicine to target two key points within the renin system, an important regulator of blood pressure. It is suitable for patients whose high blood pressure has not been adequately controlled on aliskiren alone, or by an angiotensin receptor blocker, such as Diovan, alone. It could also be considered as an initial treatment for patients who are likely to need multiple drugs to achieve their blood pressure goals.
Valturna targets the renin angiotensin aldosterone system (RAAS) in two ways. The valsartan component blocks, at the receptor level, the action of angiotensin II, which causes blood vessels to tighten and narrow. The aliskiren component directly inhibits renin, an enzyme produced by the kidneys that starts a process that leads to formation of angiotensin II. This helps blood vessels relax and open.
Because Valturna combines two different types of blood pressure drug, it offers greater blood pressure reduction than either drug would alone. It also seems conceivable having both medications available in one pill would help consumers with drug costs and insurance coverage.
Cautions: Women of child-bearing age should be particularly careful with this medicine. AVOID USE IN PREGNANCY. Do not take if you are breastfeeding or if you are planning to become pregnant. If pregnancy is detected, discontinue Valturna as soon as possible. This is because drugs that act on the renin system can cause injury and death to the unborn baby. Other serious reactions to be aware of when taking Valturna are a risk of head and neck angioedema (a rapid swelling of the flesh), and hypotension (low blood pressure). If either of these are suspected, call your physican immediately. Side effects of Valturna are the same as those of its two component medications, valsartan and aliskiren. Patients may experience tiredness, sore throat, runny nose, diarrhea, upper respiratory tract infection, urinary tract infection, flu, or dizziness. Consult your doctor before taking this or any other medication.
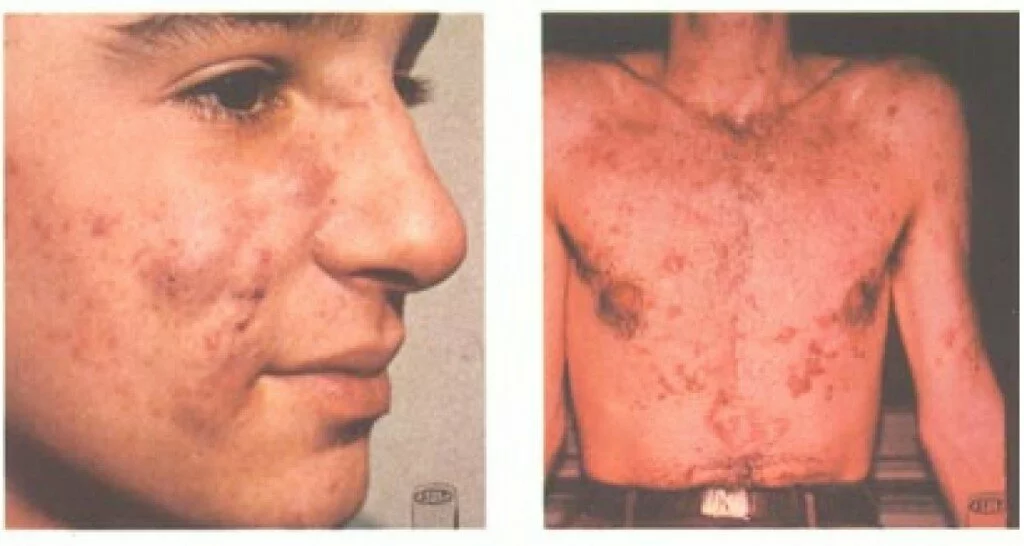 Millions of people suffer from severe, debilitating acne that does not respond to over-the-counter treatments such as acne washes and benzoyl peroxide, and even resists prescription antibiotics. Ongoing severe acne can cause physical effects such as pain, infection, and scarring, and social and emotional effects such as shame and low self-esteem. According to the Acne Resource Center:
Millions of people suffer from severe, debilitating acne that does not respond to over-the-counter treatments such as acne washes and benzoyl peroxide, and even resists prescription antibiotics. Ongoing severe acne can cause physical effects such as pain, infection, and scarring, and social and emotional effects such as shame and low self-esteem. According to the Acne Resource Center:
Of the 85% of teenagers (between the ages of 12 and 24) that suffer from acne, 25% will have permanent scars ranging from severe to light. The American Dermatologist Association finds that:
- 20% of all adults have active acne
- 60 million Americans have active acne
- 20 million Americans have acne badly enough to cause scars.
Acne affects people regardless of age, gender or race. While thoroughly treatable, of those who suffer from acne:
- 11% will see a physician
- 20% will go to a skin care center
- 30% will use an over-the-counter medication from a drug store or pharmacy
- 40+% will do nothing
The concept of 40% of sufferers doing nothing is tragic. Since 1982, we have had access to a prescription drug that treats serious cases of cystic or nodular acne. That was the year isotretinoin, under the trade name Accutane, was approved by the FDA for the treatment of severe acne. Its exact mechanism is not known, but it is thought to limit the production of sebum. Most patients see significant improvement, or remission, after a 15-to-20-week course of treatment with isotretinoin.
The drug was controversial from the start, as it is known to cause teratogenic birth defects. All physicians and patients involved with this treatment must abide by a strict pregnancy risk-management program. Isotretinoin has some other serious side effects, too. Mild side effects involve skin dryness and eye dryness, rashes, headaches, hair thinning, and backaches. More serious side effects include inflammatory bowel disease, alopecia, catacracts, blood glucose problems, and more. Because of these side effects, it is only recommended to treat the most severe cases of disfiguring acne, and patients must pay close attention to side effects.
Availability of the drug has changed this year, resulting in some confusion in consumers. Earlier this year, in July 2009, Swiss drugmaker Roche announced that it was discontinuing its sales of Accutane and moving to delist with the FDA. This was not done for safety and efficacy reasons (although Roche has had to defend itself in many lawsuits), but rather because generics of Accutane (isotretinoin) have been available since 2002 and now dominate the market. Accutane itself had only held on to a 4-5% share of market, and it did not make business sense Roche to keep selling it.
Accutane is still available in many countries outside of the United States. Within the United States, its generic form, isotretinoin, is available, and this drug is also marketed as under several different names within the United States: Claravis, Amnesteem, Sotret are a few of the best-known.
Accutane being retired in the U.S. has caused a great deal of confusion among consumers. Posts on many popular acne-related message boards reveal people trying to compare side effects and benefits of several different brand names that are all essentially isotretinoin. To clarify: there is no difference between Accutane, Sotret, Claravis, or generic isotretinoin except for their makers and packaging.
If your doctor has prescribed a cream instead of a pill, topical isotretinoin is marketed under the trade name Isotrex.
If you are one of the millions suffering from acne, know that you do have options, and your condition can improve.

Earlier this month, a closely-watched trial over the osteoporosis drug Fosamax ended in mistrial, to the frustration of nearly everyone involved. The trial was marked by great tension, with a deadlocked jury, reports of threats of physical violence, and a judge-ordered cooling-down period.
What could cause such intense drama? Well, this was just one of approximately 900 state and federal cases pending against Fosamax, alleging that that medication causes osteonecrosis of the jaw (the death of jawbone tissue). In large part, the tension in the Manhattan courtroom was that this trial — the first — was supposed to be an indicator of how these hundreds of similar cases might proceed. The other major factor is that it is notoriously difficult to “prove†drug-related injuries, and this difficulty was definitely shown in the frustration and tension among jury members.
Millions of women have taken Fosamax (alendronate), a Merck drug that was approved in 1995 to treat osteoporosis associated with menopause, and in 1997 to prevent osteoporosis itself. Until the recent introduction of some competing medications, it was one of the most popular drugs in the U.S. It is still prescribed millions of times per year to women suffering from bone loss.
In short, Merck’s defense on this topic is that there is no definitive evidence that Fosamax causes the death of jaw tissue, while plaintiffs and their lawyers insist that Merck overpromoted Fosamax without warning doctors about the potential for jaw injury. Obviously, no conclusions were reached.
Of course, this is of great interest to women who may have taken, or are considering taking, Fosamax. Only your doctor can decide what is the right choice for you, but we thought we would enumerate the other medications available for treating osteoporosis for those doing research on Fosamax alternatives. One very important thing to be aware of it is that Fosamax is not the only osteoporosis drug in this class (the bisphosphonates), which all have a similar mode of action. Fosamax is being talked about most in the media, but all are associated with some amount of risk of damaging the jaw.
If you do choose to take a bisphophonate, know these facts:
- A high proportion of jaw injuries occur following high-dose intravenous administration, so that is a particularly risky way to take the medication.
- As many as 60% of the cases are preceded by a dental surgical procedure involving the jaw. In short, women who are expecting major dental work should consider delaying treatment with Fosamax, Boniva, Actonel or similar drugs until after their dental surgery.
Here are the main alternatives on the market for Fosamax:
- Actonel (risedronate) is a bisphosphonate manufactured and marketed by Procter & Gamble and Sanofi-Aventis. It belongs to the same family of drugs as Fosamax, and may be associated with the same jaw side effects.
- Boniva (ibandronate) is also a bisphosphonate, manufactured and marketed by GlaxoSmithKline and Roche Laboratories. It is a competitor to Fosamax, and may also affect the jaw tissue.
- Evista (raloxifene) is NOT a bisphosphonate , but rather an oral selective estrogen receptor modulator from Eli Lilly and Company that affects bones through estrogen. Evista may be an interesting alternative for women concerned about their jaw health or planning oral surgery.
Also this month, The Wall Street Journal published an article called “From the Osteoporosis Front, Updates on Potential New Drugs.†These are the up-and-comers in clinical trials and going before the FDA. The story covers the latest news about Wyeth’s Viviant, Pfizer’s Fablyn, Amgen’s denosumab, and other upcoming treatments for osteoporosis.
If you are interested in following the Fosamax case that ended in a mistrial, it is expected to be re-tried in the spring. The name of the case is “In re Fosamax Products Liability Litigation, U.S. District Court, Southern District of New York (Manhattan), No. 06-1789.”
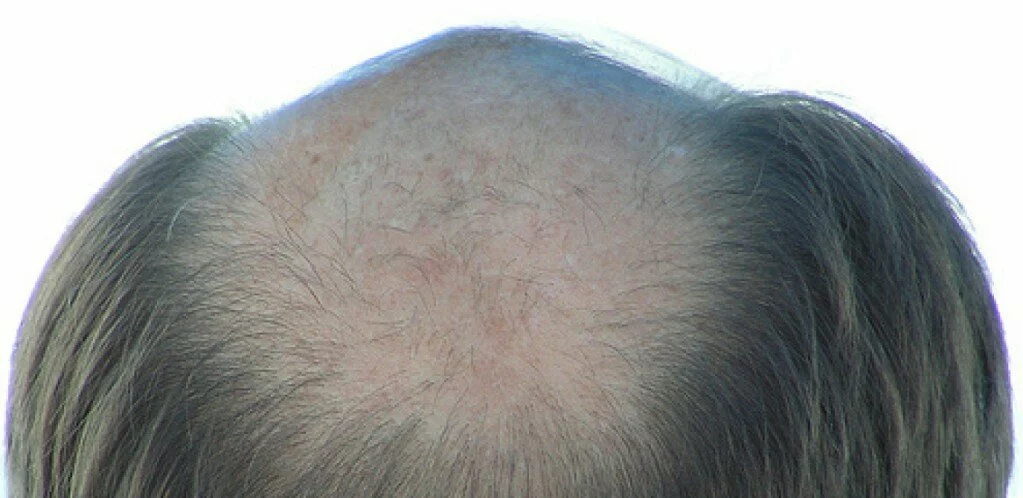
Are you one of millions of people battling some type of hair loss? More than half of males experience some degree of male pattern baldness by age 50, but even women and children can experience unwanted hair loss. There are more options than ever before to treat hair loss — such as herbal treatments, scalp massage, lasers, and surgery — but one of the most popular options is the safe and effective pharmaceuticals on the market.
There are two main medications approved by the FDA for treatment of hair loss — and they’re very different. For starters, Rogaine is a topical solution applied to the scalp, while Propecia is an orally administered pill. One medication is better than the other at treating a receding hairline. Men can use either medication, or even both, but women are restricted to just one. Because of these distinctions, it is important to choose the right hair-loss medication for you.
Let’s take a closer look at each one.
Rogaine (minoxidil) was first on the scene. Its ability to fight hair loss was discovered accidentally. The drug, a vasodilator, was originally used exclusively to treat high blood pressure, when some patients began reporting that it re-grew hair as a side effect. It was approved by the FDA in 1988 to treat male pattern baldness. Rogaine, by Pfizer, is primarily effective at stopping hair loss, but in some patients, it can increase protein blocks, which can promote new hair growth. It is said to be effective both on the hairline and vertex of scalp. Rogaine can be used by both men and women, in a 2% or 5% solution.
Propecia (finasteride), made by Merck, is an orally administered medication approved by the FDA in 1997 to treat hair loss. Unlike Rogaine, which is a vasodilator, Propecia acts on hair loss through hormonal means. (Because of this, Propecia should NOT be used by women or children; it could be very dangerous.) Propecia is an anti-androgen, which decreases the conversion of testosterone to dihydrotestosterone (DHT), a chemical responsible for balding. With DHT inhibited, existing hair is better maintained, and the body can put more energy into thinning follicles so that they become thicker. Propecia has high effectiveness with early to moderate hair loss, and works best on the crown of the head, but not as well with a receding hairline.
Neither Rogaine nor Propecia is a quick fix for hair loss. They both need to be taken for long periods. It can take anywhere from 6 to 24 months to see initial results, and patients may need to take their chosen medication indefinitely keep treating the condition.
Knowing all this, which factors should guide your choice between Rogaine and Propecia?
- Your gender: if you are male, you can be prescribed either (or both), but if you are a woman, you can only use Rogaine.
- Your goals: Rogaine is slightly more useful for retaining existing hair, while Propecia is said to be more effective at promoting new hair growth.
- Your area of hair loss: Propecia has good results mainly on the crown, while Rogaine has documented success on the hairline and the crown.
- Medical interactions: Consult your physician to determine which drug is a better fit for your personal health conditions and other medications.
- Side effects: With Propecia, you may experience decreased libido or gynecomastia. Rogaine may trigger allergic effects, chest pain, dizziness, or irregular heartbeat.
- Ease of use: Some people feel that a daily pill is simpler than a twice-daily application to the scalp, but others prefer to pick their medication based on its mode of action.
Whichever method you select, be patient, and remember to keep feeling good about yourself, your hair, and your overall health. As with any drug, please consult your physician before you begin any medication.

Painful conditions of the esophagus and stomach are common in our society. Stress, obesity, poor diet, and smoking can all play a part. Whether you suffer from acid reflux or ulcers, controlling acid is probably part of your game plan for comfort and health. But there are two very similar acid-controlling drugs from the same parent company on the market: Prilosec and Nexium. How does a savvy healthcare consumer evaluate which one is right or them?
The basics:
Prilosec (omeprazole) was introduced in 1989. Nexium (esomeprazole) was released in 2001. These two drugs are very similar chemically — they are both proton pump inhibitors. This means inhibit the secretion of hydrochloric acid. Yet, they are subtly different: Nexium is often described as a “reflected†or “left-hand” version of Prilosec. They are both products of the pharmaceutical company AstraZeneca, and some parties have described Nexium as a “follow-up drug†to the wildly successful Prilosec; it was introduced just as Prilosec’s patent protection was about to expire.
The conditions they treat:
- Gastroesophageal reflux disease (GERD, commonly called acid reflux) is when the lining of the esophagus becomes inflamed from the regurgitation of stomach acid. Prilosec and Nexium can both treat GERD.
- Duodenal ulcers: Prilosec and Nexium can both treat duodenal ulcers that are caused by a Helicobacter pylori infection, but only Prilosec can treat duodenal ulcers caused by other factors.
- Gastric ulcers: Both drugs treat benign gastric ulcers, but Nexium is limited to treating such ulcers caused by nonsteroidal anti-inflammatory drugs. Prilosec can treat any type of gastric ulcer.
Your choices:
Prilosec is an older, more established drug, and thus has been approved to treat more conditions than Nexium has. If your condition is one that only Prilosec can treat, then your choice is crystal-clear.
However, if you are one of the millions of people whose condition is approved to be treated with either Prilosec or Nexium, then you have some research, discussion, and possibly even a trial run with the medication ahead of you. Someone suffering from acid reflux, for instance, would have a valid case to try either medication. If this is the case, discuss your particulars with your doctor for advice on which to start with.
The two drugs are so similar that many patients report choosing solely based on logistical factors such as availability and affordability. However, here are some other issues to consider in your comparison:
- Check your insurance plan: some cover both Prilosec and Nexium, while some only cover one choice.
- Prilosec has a generic version, while Nexium does not, which may affect your buying decision.
- Some people tolerate one drug better than its sibling; you may need to compare to learn what works for you.
- Prilosec can have a side effect of appetite loss, while this has not been reported with Nexium.
- Prilosec is generally recommended for shorter courses of treatment, while Nexium is often recommended for longer courses of 4-8 weeks.
These medications are very similar, but are not the same. Do not substitute. As with any drug, please consult your physician before taking.
A diagnosis of high cholesterol can be intimidating, but there is a lot you can do to control this condition. In addition to modifying your diet and upping your exercise, the addition of a HMG-CoA Reductase Inhibitor — a class of drugs commonly called “statins†— can safely and effectively lower your cholesterol. (HMG-CoA Reductase helps our liver produce cholesterol; when the chemical is inhibited, the amount of cholesterol is correspondingly reduced.) For people with heart disease, statins can lower the risk of a cardiac event and subsequent death. If you and your doctor have determined that you need a statin, how can you pick the right statin for your needs?
There are six statins on the market: atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin. They differ in their ability to reduce cholesterol, and they also differ in their rates of reducing heart attacks. Their costs are also quite different — and since most people take statins for a long time, the costs add up over the years. With all of these variables, choosing the right statin for you can be complex.
All statins are capable of lowering LDL (“badâ€) cholesterol and triglycerides, and raising HDL (“goodâ€) cholesterol. The statins do differ in how effectively they can do this, and it is highly dose-dependent. Says Drug Digest:
If the needed LDL-C reduction is up to 35-36%, any of the statins should be acceptable choices for therapy. For a desired reduction of LDL-C greater than 42%, simvastatin (Zocor), atorvastatin (Lipitor), or rosuvastatin (Crestor) would be needed.
Indeed, the best-known statins are Crestor, Lipitor, and Zocor (quite probably because they have the greatest effect on cholesterol levels). The latter two are also endorsed by Consumer Reports. Taking evidence for effectiveness, safety, and cost into account, the publication rated both of these statins as “Consumer Reports Best Buy Drugs.†They recommend:
• Generic simvastatin (20mg or 40 mg) — if you need 30% or greater LDL reduction and/or have heart disease or diabetes, or if you have had a heart attack or have acute coronary syndrome and your LDL level is not highly elevated.
• Atorvastatin (Lipitor) (40mg or 80mg) — if you have had a heart attack or have acute coronary syndrome and your LDL is highly elevated; use for two years and then reconfirm need or switch to generic simvastatin.
Charts on Drug Digest have some great comparisons. For instance, they show that Lipitor (10-80 mg.) can reduce total cholesterol by 25-45%, while Zocor (5-80 mg.) can reduce the same numbers by 19-36%, and Crestor (5-40 mg.) can reduce it by 33-46%. As for lowering HDL, Lipitor can offer reduction of 5-9%, Zocor lessens HDL by 8-16%, and Crestor lowers these numbers by 8-14%. As you can see, choosing the proper statin has a lot to do with which numbers (Total Cholesterol, HDL, LDL, or triglycerides) you are trying to effect.
A final consideration is that last year there was reporting on an observational study done by Pfizer that suggested that there were certain benefits to using Lipitor over Crestor. However, one must keep in mind that Pfizer conducted the study, and they are the manufacturer of Lipitor, and they are defending this drug against Merck’s Zocor product, which is now available in a generic formula. Here is the information as presented by The Wall Street Journal:
An analysis, published in the latest Clinical Therapeutics Journal, mined a large database of health-care records and found that patients taking Lipitor had a 12% lower risk of a cardiovascular event than those on simvastatin, the generic name for Zocor. The patients on Lipitor had a 15% lower risk of having a heart attack.
So-called observational studies like this one that look at data after the fact aren’t as powerful as prospective clinical trials. Jack Tu, a cardiologist who specializes in outcomes research at Canada’s Institute for Clinical Evaluative Sciences, says the latest Pfizer study didn’t take into account factors that could predispose a patient to heart problems, such as smoking and cholesterol levels. “Just on this alone, you wouldn’t recommend that everyone should switch onto Lipitor,†he says.
Still, Pfizer hopes that doctors will take notice. “We’ve done two rather large observational studies and patients have a lower risk of cardiovascular events on Lipitor [compared with] simvastatin,†says Susan Shiff, Pfizer’s team leader for cardiovascular outcomes. “Doctors need to factor this into discussions with patients.â€
You should definitely discuss with your physician which statin is right for you. In general, the best plan is to take the LOWEST dose of a statin that gets you to your target level for cholesterol. Overly large doses can be harmful to your liver and to your muscles. If you experience muscle aches and pains when taking a statin, contact your doctor immediately.
-
Search Blog Posts
-
-
Trending Content
-

-
Blogroll
- Bullet Wisdom
- Christian Social Network
- DrugWonks.com
- Eye on FDA
- GoozNews
- Health 2.0
- In the Pipeline
- Jesus Christ Our King
- Kevin, M.D.
- Pharm Aid
- Pharma Marketing
- PharmaGossip
- Pharmalot
- San Antonio Asphalt
- San Antonio Life Insurance
- San Antonio Pressure Washing
- The Angry Pharmacist
- The Health Care Blog
- The Peter Rost Blog
- World Vision
-
Tags
big pharma Canadian drugs canadian pharmacies canadian pharmacy consumer reports craig newmark divine healing Drug costs drug prices Drug reimportation eDrugSearch.com FDA Fosamax Generic drugs healing scriptures Health 2.0 healthcare reform Hypertension Jehova Rophe Jesus Christ Lipitor Metformin miracles nabp online pharmacy dictionary online prescriptions osteoporosis peter rost Pharmacies pharmacists pharmacychecker pharmacy spam phrma Prescription drugs prescription medication Proverbs 3:5-8 reimportation relenza Roche saving money SSRI swine flu Tamiflu The Great Physician The Lord our Healer -
Archives
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- August 2012
- July 2012
- June 2012
- April 2012
- March 2012
- February 2012
- January 2012
- November 2011
- June 2011
- August 2010
- July 2010
- June 2010
- May 2010
- April 2010
- March 2010
- February 2010
- January 2010
- November 2009
- October 2009
- September 2009
- August 2009
- July 2009
- June 2009
- May 2009
- April 2009
- March 2009
- February 2009
- January 2009
- December 2008
- November 2008
- October 2008
- September 2008
- August 2008
- July 2008
- June 2008
- May 2008
- April 2008
- March 2008
- February 2008
- January 2008
- December 2007
- November 2007
- October 2007
- September 2007
- August 2007
- July 2007
- June 2007
- May 2007
- April 2007
- March 2007
- February 2007
- January 2007
- December 2006
- November 2006
-
Recent Comments
- Ranee on What is the Difference Between Effexor and Cymbalta?
- bAnn805 on Crestor, Lipitor, or Zocor – Which statin is right for you?
- Anna Pham on Why is Medicine Cheaper in Canada?
- Anna Pham on How to Get the Cheapest Prescription Medications
- Anna Pham on How to Travel With Prescription Medication
